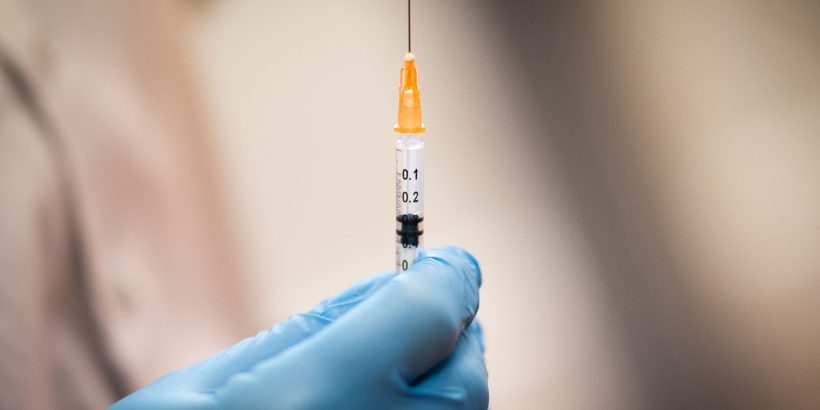
The vaccine become the third approved for use in the United States.
By
The Food and Drug Administration on Saturday announced it had approved the Johnson & Johnson COVID-19 vaccine for emergency use throughout the country.
In a press release announcing the decision, the agency said it had “determined that the [vaccine] has met the statutory criteria” for an emergency authorization.
“After a thorough analysis of the data, the FDA’s scientists and physicians have determined that the vaccine meets the FDA’s expectations for safety and effectiveness appropriate for the authorization of a vaccine for emergency use,” Peter Marks, the director of the FDA’s Center for Biologics Evaluation and Research, said in the announcement.
Bookmarks:
*C-VINE-MeWe, https://mewe.com/join/C-VINE
*C-VINE-Rumble, https://rumble.com/user/CVINE
*C-VINE-GAB, https://gab.com/groups/6078
*C-VINE-Parler, https://parler.com/profile/CVINE/posts
*C-VINE-Telegram, https://t.me/CVINENEWS
*C-VINE YouTube Channel, https://www.youtube.com/c/CVINENewsNetwork/videos
Facebook Groups :
*C-VINE Commentary & Analysis
https://www.facebook.com/groups/895771901163001
*C-VINE Business Network, https://www.facebook.com/groups/1242625425864710
*C-VINE Natural Health News Network, https://www.facebook.com/groups/cvinehealth
*C-VINE Patriot Prayer Brigade, https://www.facebook.com/groups/482643725948621